Is Sugar Solution a Good Conductor of Electricity
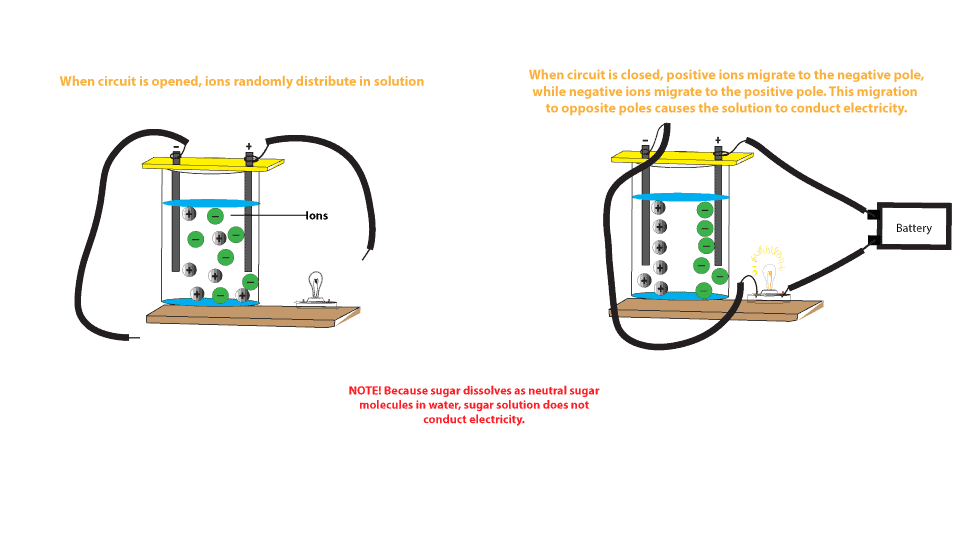
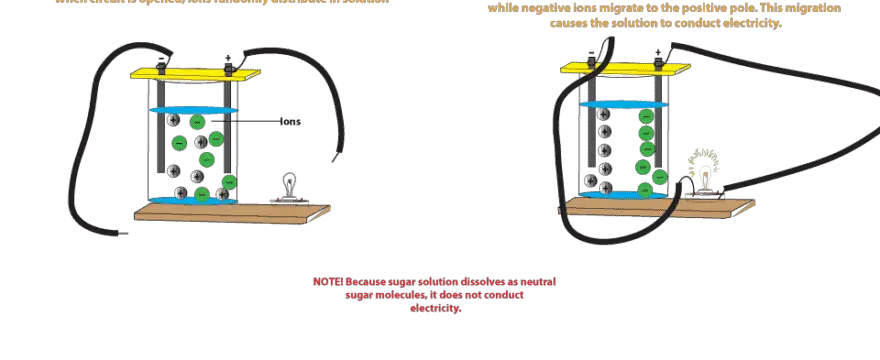
The sodium chloride solution mainly consists of free sodium and chloride ions which could migrate to positively charged electrodes. On the other hand sugar solution does not conduct an electric current because sugar C 12 H 22 O 11 dissolves in water to produce sugar molecules.

Explain Why A Solution Of Cane Sugar Does Not Conduct Electricity But Solution Of Youtube
Sugar solution is not a good conductor of electricity.

. In the solution it does not have ions to conduct electrical charges across the solution. Is glucose a good conductor of electricity When glucose is added to water it readily dissolves but it does not dissociate into ions. Common salt is an ionic compound containing ionic bonds whereas cane sugar is a covalent compound containing covalent bonds.
That being said water undergoes a process called self-ionization in which. The neutral molecules do not have any charge and thus do not conduct electricity. These sugar molecules are usually neutral not charged and so are unable to move to the opposite ends of the electrodes like the ions.
Hence it does not conduct electricity. Some substances that are made of. When sugar is dissolved in water it does not create ions in water.
These sugar molecules are usually neutral not charged and so are unable to move. Sugar solution is a good conductor of electricity. Does sugar conduct electricity why.
A sugar solution is not a good conductor of electricity. Therefore the glucose solution is a non-electrolyte and is a poor conductor of electricity. This water is thus a good conductor of electricity.
Why is sugar water NOT a good conductor of current. Sugar does not ionize when dissolved in water so it does not produce ions that are necessary to conduct electricity. There are no free-to-move electrons in a solution e.
Therefore it is not an electrolyte solution that can conduct electricity. Small amounts of mineral salts are naturally present in it. When this seawater is used as an electrolyte solution the cations and anions move to the opposite electrode and conduct electricity.
Play this game to review Physics. When sugar is dissolved in water the solution does not conduct electricity because there are no ions in the solution. Sugar is a nonconductor.
When it dissolves into water it dissolves as a covalent molecule. Thus sugar solution is a bad conductor of electricity. A sugar solution is a nonconductor because no ions are present c.
Sugar water contains no electrons. On the other hand sodium chloride solution contains free mobile ions and allows electric current to pass through it. Distilled water contains only 10-7 molL of H and 10-7 molL of OH-.
On the other hand sugar solution does not conduct an electric current because sugar C12H22O11 dissolves in water to produce sugar molecules. When it is dissolved in water does not dissociate to give free ions which could migrate to cathode or anode. It may contain several salts dissolved in it.
When sugar is dissolved in water sugar does not dissociate into ions. If by sugar you are referring to table sugar sucrose it does not conduct electricity to any appreciable extent when dissolved in water. A sugar solution is formed when sugar is dissolved in water.
An electric current is a movement of electric charge d. Water can conduct electricity only when charged ions are present in it. No sugar does not conduct electricity because it can not ionize so it does not carry a chargein the matter of dissolving it in water.
Cane sugar is compound which does not have ions even in solution and contains only molecules. As a covalent molecule it does not conduct electricity in the way that ionic compounds like salt would. The sugar cane solution is a covalent compound.
Since ionic compounds conduct electricity and covalent compounds do not hence common salt is a good conductor of electricity and cane sugar is a non-conductor of electricity. Here is a model that illustrates why NaCl conduct electricity when dissolved in. Yes but no more than distilled water.
Cations in a solution move toward the positively charged electrode 17. So seawater is not a bad conductor of electricity rather a good conductor of electricity. Hence sugar solution is bad conductor of electricity.
Is sugar solution a conductor of electricity. The water that we get from sources such as taps hand pumps wells and ponds is not pure. It is a non electrolyte.
A solution of cane sugar does not conduct electricity because cane sugar is a covalent compound which is bonded by the sharing of electrons. Thus a sugar solution contains only neutral molecules of sugar and water.

Does Sugar Solution Conduct Electricity Techiescientist
Why Does Salt Solution Conduct Electricity While Sugar Solution Doesn T
Why Does Salt Solution Conduct Electricity While Sugar Solution Doesn T
No comments for "Is Sugar Solution a Good Conductor of Electricity"
Post a Comment